22 Predict Which of the Following Has an Ionic Bond
D all of the above. 7 - Predict which of the following compounds are ionic.
Problem 39 says to predict whether a bond between the indicated Adams and A Through F is likely to be Ionic Co.
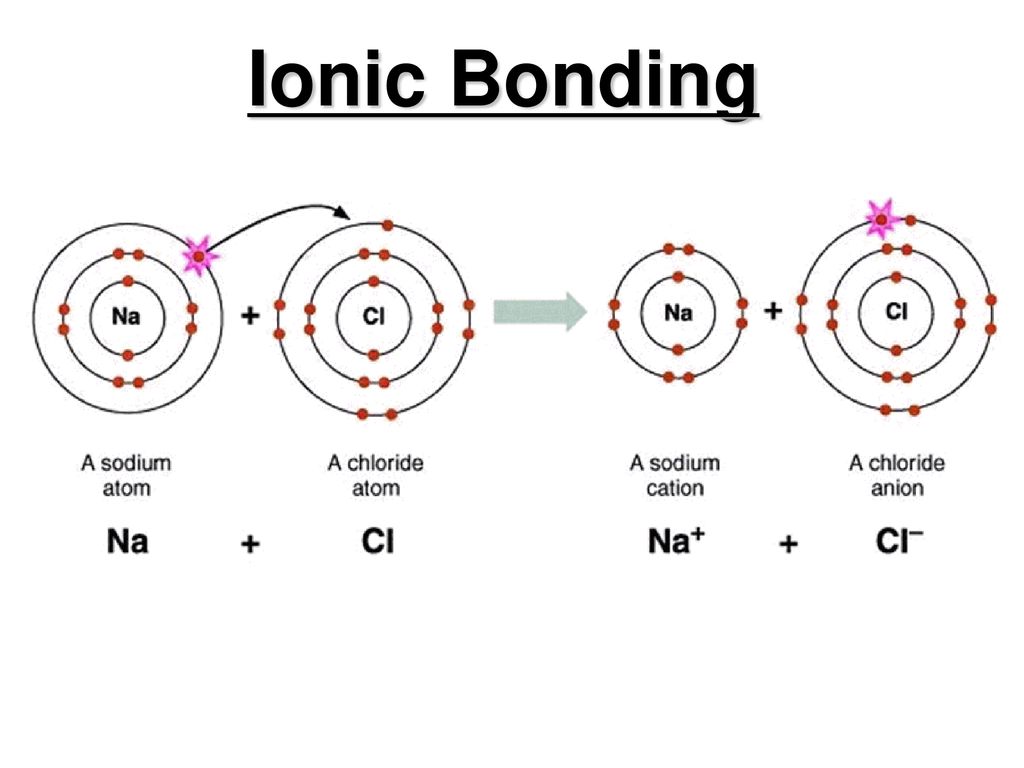
. Vaillant Bond is formed between non metals with similar electro negativity. A PCl 5 B CrCl 6 C RbCl D PbCl 2 E NaCl. If one atom is on the far left Group 1 or 2 and the other is on the far right Group 5 6 or 7 then the atoms will have large differences in EN and will form an ionic bond.
B resists a change in pH. Vaillant or Polar Covalin. 1 Predict which of the following has an ionic bond.
Problem 39 says to predict whether a bond between the indicated Adams and A Through F is likely to be Ionic Co. These two chlorine atom generally shares each other electrons and for. Which of the following statements about ionic compounds are true.
Thus we can conclude that the compound that has the smallest ions with the greatest charge would be expected to have the strongest ionic bonds. When electronegativity difference is greater than 04 and less than 18 then bond between the two atoms is a polar covalent bond. So if we have elements with a large electric negativity difference between them then we form ionic compounds.
Therefore smaller is the size of ions greater will be its charge and more strong the ions will be held together. One way to predict whether a bond is ionic or covalent is to look how far apart the two atoms forming the bonds are in the periodic table. The ionic radius of an iron ion is equal to its atomic radius.
Si-O 190 - 344 154. These electrons are simultaneously attracted by the two atomic nuclei. 22Which pair of elements is most likely to form an ionic compound with each other.
None of the above. The ions in a crystal lattice are arranged randomly and the overall charge is zero E. Sugar is a ionic bond because hydrogen and carbon are opposite charges that formed sugar.
D removes hydrogen ions from a solution when the pH increases. View the full answer. Predict whether each of the following is held together by ionic or by covalent bonds.
Predict which of the following has an covalent bond. Thats definitely an Ionic bond. Whereas elements with quite small election negativity differences for covalin compounds.
But if the EN. Vaillant or Polar Covalin. A keeps the pH of a solution neutral.
E none of the above. OOOOOOOO Electronegativity difference can be used to predict bond type. When you dissolve ionic bonds in water they can conduct electriciry because the ions are free to move now.
Most ionic compounds are insoluble in water D. All of the above e. Predict which of the following has an ionic bond.
Using the periodic table predict whether the following compounds are ionic or covalent. Breaking an ionic bond between Fe2 and S2- releases energy. D all of the above.
None of the above. A buffer is a chemical that. A chemical reaction takes place when.
B resists a change in pH. Which of the following statements is true regarding an ionic bond in FeS. A H 2 S B NH 3 C CH 4 D all of the above E none of the above.
2 Predict which of the following has an ionic bond. Up to 256 cash back An ionic bond results when the sharing is so unequal that fully charged ions form. All of them are nonmetals-which dont conduct electricity Potassium Hydroxide is an ionic bonded compound because it has oxygen and potassium that bonded together.
2 has ionic bonds. Its remember that in general Ionic bonds are formed between a metal on a non metal ako. A barium Chlorine B calcium sodium C oxygen fluorine D sulfur carbon E nitrogen hydrogen 23Of the choices below which one is not an ionic compound.
Ionic compounds have high melting points B. The bond which is formed between a metal and a non metal is known as ionic bond. If the electronegativities differ by less than 04 units the bond can be classified as nonpolar covalent.
D all of the above. A aluminum oxide Al. D Al 2 SO 4 3.
And so then theres also polar Co Vaillant with the difference in electro negativity of about 04 aluminum and oxygen aluminum sometimes considered a metal Lloyd. 7 - Explain the difference between a nonpolar covalent. One method to classify bonds based on this difference can be described as follows.
1 H2Se 2H2S 3 H2O 4 all of the above 5 none of the above. If the Electronegativity Difference EN18 then the compound will be ionic. 7 - From its position in the periodic table determine.
A covalent bond consists of the mutual sharing of one or more pairs of electrons between two atoms. Iron and sulfur ions bond by electrostatic attraction. Covalent bonds occur between identical.
Its remember that in general Ionic bonds are formed between a metal on a non metal ako. 3 Predict which of the following has an ionic bond. Sze and a puller Co Vaillant bond as between two non metals with.
Iron atoms gain electrons and sulfur atoms lose electrons. Predict which of the following has an ionic bond. In NaCl the electronegativity of sodium chlorine are 093 316 respectively.
Ionic compounds have high. Ionic compounds can be solid liquid or gas at room temperature C. But we think of it as a metal with oxygen thats a non metal.
7 - From their positions in the periodic able arrange. 3 b magnesium nitride Mg. Predict which of the following has an ionic bond.
3 b sulfur dioxide SO. C N 2 H 4. Electronegativity difference of the given molecules is as follows.
When electronegativity difference is 18 or greater than the bond formed is ionic in nature. Predict which of the following has a covalent bond. 7 - From its position in the periodic table determine.
But theres a difference in electro negativity so it would be polar Co. Answer- 8 LiCl - LiCl shows an ionic character. E none of the above.
Predict whether each of the following is held together by ionic or by covalent bonds. So typically if we have a metal and non metal that will give us an ionic compound and if we have to non metals then well get a Covalin compound. C adds hydrogen ions to a solution when the pH decreases.
- It has non polar covalent bond between them. Vaillant Bond is formed between non metals with similar electro negativity. A chemical bonds are formed.
Form the molecular formula you can compare the Electronegativity EN between the atoms. Which of the following describes the share of a non-bonding electron pair on a N molecule with an O atom resulting in a nitrous oxide molecule.

Ionic Bond Examples Biology Dictionary


0 Response to "22 Predict Which of the Following Has an Ionic Bond"
Post a Comment